More of the world’s medicines could get made in India, if it can cure its pharma ills
India wants a bigger slice of the world’s pharmaceutical pie amid US-China tensions. But with its patchy record on safety and a looming deadline to raise quality controls, the programme Insight explores whether the country can step up.
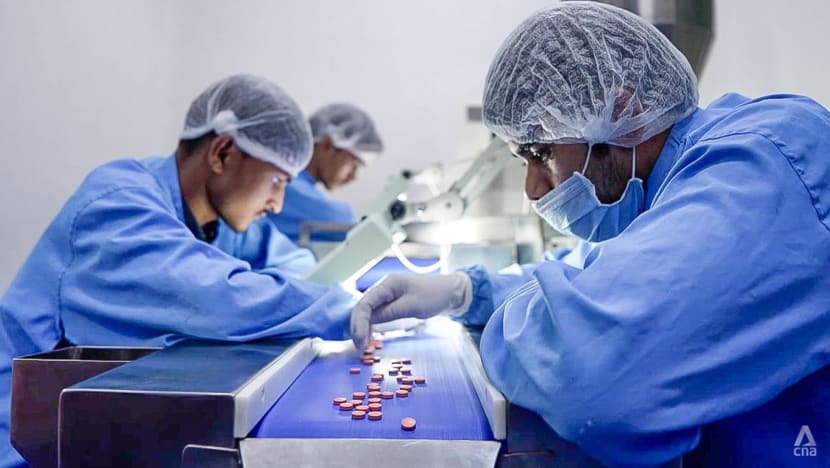
Workers carrying out quality control checks on pills in an Indian pharmaceutical manufacturing firm.

This audio is generated by an AI tool.
MUMBAI: Amid a rash of drug-related recalls and deaths, India’s pharmaceutical sector is racing to improve its quality and safety standards as it eyes a greater share of the global market.
Just last year, toxic cough syrups from India were linked to the deaths of at least 141 children in Cameroon, Gambia and Uzbekistan.
According to the World Health Organization (WHO), in a , at least 15 countries may have had these contaminated syrups on sale.
In 2022, when Indian manufacturer Intas Pharmaceuticals failed the United States’ quality control benchmarks, the company temporarily suspended its production of the cancer treatment drug, cisplatin, triggering a shortage and sending the US’ healthcare sector scrambling for alternative supplies.

“(In the) earlier days, there were no regulations (in India). And regulations were only on paper,” said Indian Pharmaceutical Alliance secretary-general Sudarshan Jain. “Now we’ve got Schedule M, which is very close to the global market (standards).”
To improve standards and expand India’s pharma industry, the Modi government revised Schedule M, a set of rules under the Drugs and Cosmetics Act, last December.
Drug manufacturers currently have until the year end to align their quality controls more closely with the WHO’s Good Manufacturing Practices (GMP) for pharma products. Doing so would allow more small and medium Indian firms to enter the export market.
And with a possible decoupling between the US and China’s biotech industries, the South �鶹��ýn country could gain a greater foothold in the global pharma space.
But with little time left to clean up their act, many small and medium local firms are seeking a two-year extension of the deadline. With its eye on increasing exports, can India cure its pharma ills, the asks.
WATCH: Bad medicine — Why India is racing to improve pharma standards amid US-China trade rivalry (46:58)
BAD DRUGS, BAD ACTORS
According to experts, India’s pharma industry faces three main problems. The first is counterfeit drugs.
In September, the country was rocked by a scandal over antibiotics supplied to government hospitals that were found to be made from talcum powder and starch. Further investigations showed that a syndicate was making and selling fake medicines across India.
The second problem is adulterated drugs, such as the cough syrups that caused the deaths of children beyond India’s shores.
And the problem is not new. Cough syrups made by Indian companies also came under scrutiny after the deaths of 12 children in Jammu and Kashmir between December 2019 and January 2020.
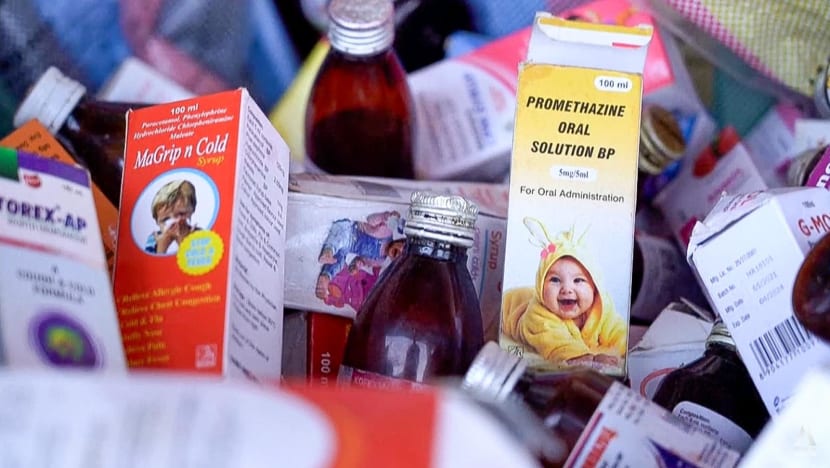
In such cases, very likely manufacturers had substituted glycerine — a common ingredient in cough syrups — with diethylene glycol, according to T Prashant Reddy, co-author of the book, The Truth Pill: The Myth Of Drug Regulation In India.
Diethylene glycol is used for industrial purposes, for example in antifreeze and brake fluids. Even low doses can be toxic.
The third problem — India’s most pervasive one — is substandard drugs.
“This may be a tablet, for example, that has only 50 per cent of the active ingredient that’s there on its label,” said Prashant. “Or it may be a tablet that doesn’t dissolve properly in your blood.”
Although India’s medicines have the same generic names, for example paracetamol, the quality can be inconsistent. Different manufacturers may use different formulations of active ingredients and excipients (stable additives used so that a drug is suitable to administer).

In financial year 2022 to 2023, more than 3.5 per cent of drugs tested by India’s drug regulator were found to be of non-standard quality or spurious.
Last year, the Indian pharma industry recalled 66 products from the US. And this June, India’s regulator said more than 36 per cent of the 400 drug-making units inspected since last year were non-compliant and ordered to be shut.
PRICE PRESSURES, REGULATION ISSUES
With nearly 88 per cent of Indian companies in the pharma trade comprising micro, small and medium enterprises, the problem is that they often face pressure to keep prices low, driven by the intense competition.
So they may skimp on quality materials during the manufacturing process or forgo quality testing at every stage of production, transport and storage.

“To cut down (on) costs, (some) companies may (be) going for technical grade (materials) rather than going for an IP grade,” said Radico Remedies chief executive officer Vikramjit Singh Sawhney.
IP refers to the Indian pharmacopoeia, a compilation of the standards for drugs manufactured in India.
According to the IP, drugs must be tested at each stage of manufacturing. But testing can be expensive and can eat into 25 to 40 per cent of a pharma unit’s overall turnover.
“Many companies … were (either) not familiar with upgrading or they weren’t able to do it,” said Dagon Pharmaceuticals director Gautam Bhut. “The problem is that it requires a lot of investment.”
Then there is the nationwide shortage of drug inspectors and testing capacity.

In the talcum powder case, a government laboratory in Mumbai took nine months to analyse the antibiotic samples sent for testing. The same lab has more than half of its staff positions unfilled, the Indian Express reported in October.
Maharashtra state’s drug inspection unit is also reportedly functioning at 40 per cent of its staff strength.
D R Gahane, joint commissioner of the Maharashtra Food and Drugs Administration (FDA), has had to instruct inspectors to focus on drugs “mainly used (by) the common people” and restrict the number of samples drawn as per the laboratory’s testing capacity.
The “meagre regulations” India has, described Prashant, are “very poorly enforced” by drug inspectors.
Regarding the deaths linked to the country’s toxic cough syrups, Dinesh Singh Thakur, fellow co-author of The Truth Pill, highlighted that no party has been held accountable so far.
WATCH: The pharmaceutical companies behind cough medicine deaths in children | Undercover �鶹��ý (46:46)
AN OPPORTUNITY TO STEP UP
Despite the scandals, India’s pharma industry is booming and is projected to double in value to US$120 billion to US$130 billion by 2030.
Dubbed the “pharmacy of the world” by Prime Minister Narendra Modi, the country is the third-largest drug manufacturer by volume.
It is the world’s largest supplier of generic drugs, with a 20 per cent share of the generics market. It also makes half of the world’s vaccine doses.
And with geopolitical developments elsewhere, an upgrade in the standards of India’s pharma sector could be more lucrative than expected.

In September, the US House of Representatives passed the Biosecure Act over national security concerns about American firms relying heavily on Chinese contract manufacturers to develop their therapeutics.
“The worry is that biosecurity can be weaponised and be used as an instrument of war,” said Benjamin Ho, an assistant professor at the S Rajaratnam School of International Studies.
When pharma firms conduct clinical trials, they gather individual health information that “can be exploited”, highlighted Centre for �鶹��ý Pacific Strategy vice president David Maxwell.
If the Act is signed into law, American pharma companies would be barred from working with five named Chinese firms. And should the US reduce its reliance on Chinese biotechnology, India could have an opportunity to fill the gap.
The US is the world’s largest pharma market. And its fourth-largest supplier of medicines is China, which has also supplied, over the past decade, 17 per cent of its active pharmaceutical ingredients, the active components of drug products.
India, meanwhile, has about 650 USFDA-approved plants, the highest number outside the US. Drugs made here have a different quality threshold than drugs made for the domestic market, which Thakur calls “dual-track manufacturing”.
When producing water for injections for the US, he cited, the Indian manufacturers ensure that the water goes through the necessary filtration process. “Guess what they use for formulating injectables for consumption in India? Tap water,” he said.
To get a bigger slice of the global market, however, standards in India’s entire pharma sector must be elevated.

Before the revision of the Schedule M rules, only about 2,000 of the country’s 10,500 pharma manufacturing units had the WHO’s GMP certification.
And to meet the new standards such as in quality testing, costs will be incurred, said Sawhney — which is why some smaller manufacturers have petitioned to extend the deadline for compliance.
INCREASED STANDARDS NOT ENOUGH
Even if India improves its standards, this alone will not guarantee its competitiveness as a world pharma powerhouse, say the experts.
Without investing in more innovation and talent, India risks being left behind as global markets shift towards more advanced drugs.
“If you look at the drug supply for the future, it’s all biologics and biosimilars,” said Thakur. “Our capacity for biosimilars is miserable. We’ve never invested.”
Biologics are pharmaceuticals made from living cells or organisms instead of chemicals; biosimilars are biological medicines that are similar to their reference products.
“If an even larger market shifts (towards that), then we’re going to be left behind,” said Dinesh Abrol, a professor in Jawaharlal Nehru University’s Transdisciplinary Research Cluster on Sustainability Studies. “China will be ahead of us.”
Last year, China filed almost 20 per cent of global biotech patents, the highest in the world. The median investment in research and development was about US$240 million for Chinese biopharma companies and about US$90 million for their Indian counterparts.
Moreover, India’s talent pipeline is not keeping up with its pharma sector’s growth. In a 2022 government survey, almost 8 in 10 companies reported recruiting people without hard pharmaceutical experience to save costs. “We don’t have biology skills,” asserted Thakur.
.png?itok=ot3HsuCi)
So it may not be that easy for the US to decouple from China and for India to fill the gap after all.
During the cisplatin crisis, for example, the USFDA turned to a Chinese drugmaker to make up for the shortfall from India.
Most of the experts Insight spoke to agreed that India’s pharma industry has a long way to go before it can reach its full potential.
“On paper, certain regulations have been (put) into place. But we have serious doubts as to whether any of those regulations are enforced on the ground,” said Prashant.
“The industry is booming. Exports are booming. Where’s the incentive to fix this? Domestically, no one’s bothered about this issue, apart from a few of us.”
Thakur added: “It’s great to kind of market yourself as the pharmacy of the world. … But peel the layers a little bit and start looking. The reality is something different.”
Watch this episode of . The programme airs on Thursdays at 9pm.












